
Disclaimer: I am a full-time employee of Bristol Myers Squibb.
When I practiced clinical rheumatology, I would often see patients with autoimmune conditions like systemic lupus erythematosus (SLE), systemic sclerosis (scleroderma), rheumatoid arthritis (RA), or myositis. A typical patient journey included an initial sense of relief when a diagnosis was established and a medicine was started. Inevitably, however, there was a sense of dread when a patient would ask: “When am I able to stop taking these strong immunosuppressive medicines?”
I would answer: “Likely never.”
That’s because, historically, there have been no cures for these diseases.
Now, however, I believe there may be an opportunity for new strategies that may deliver transformational outcomes for patients. In a new review paper published in Nature Reviews Drug Discovery (NRDD) (link here), our team at Bristol Myers Squibb (BMS), led by Dr. Francisco Ramírez-Valle, describes a “sequential immunotherapy” strategy that has the goal of achieving durable remissions and even functional cures. I presented an example of this strategy in action for SLE at the Stanford Drug Discovery Symposium. I also discussed the potential for functional cures during a recent BioCentury Show podcast and as part of NRDD’s “An Audience With” series. Importantly, this sequential immunotherapy strategy was originally developed by Dennis Zaller during our time working together at Celgene (see also addendum below).
In this short blog, I briefly describe the concept of sequential immunotherapy, and why I believe – for the first time – there is the potential for functional cures in SLE, scleroderma, myositis, and a variety of other autoimmune diseases, and spanning into neuroscience with diseases like multiple sclerosis (MS). For the details, you will need to dig into the NRDD review, which is >11,000 words and contains >350 references! But trust me…the paper includes many important points, so it’s a worthy investment of your time.
I want to first start with the question: What is the evidence that functional cures are possible? There are several lines of evidence that include nature’s experiments (e.g., clinical remission observed with pregnancy or liver transplants), together with data from autologous hematopoietic stem cell transplant. In these settings, clinical remission – defined by the absence of disease activity following the removal of all immunosuppressant medications – has been achieved.
Our sequential immunotherapy strategy outlines how functional cures can be achieved through pharmacological intervention. The goal is to re-balance the immune system from a state where immune cells recognize “self” (i.e., autoimmunity) to a state where immune cells only recognize foreign antigens (e.g., infections, tumor cells). Immunologists like to use the teeter-totter visual, as shown here:
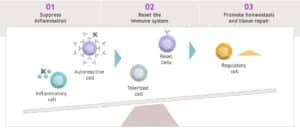
If there are too many inflammatory or autoreactive cells, the teeter-totter tilts left, leading to autoimmunity. This is the state shown in the Figure above. If these inflammatory cells can be controlled through targeted therapy (Step 1), autoreactive cells eliminated through tolerization or depletion (Step 2), and regulatory cells increased through T regulatory cell expansion (Step 3), then a healthy state of immune homeostasis can be achieved, and the teeter-totter normalizes or even tilts right.
We use the term sequential immunotherapy, as it reflects the typical patient journey. A patient presents with symptoms, which must be controlled first by suppressing the immune system. As readers of this blog know, one of my favorite “Step 1” targets is TYK2 (link to previous blogs here).
What is increasingly exciting about Step 1 therapies is the potential for targeted therapy in specific patient populations. There is a lot of research in defining specific subsets of patients with SLE (link here) and other autoimmune diseases, and this provides an opportunity to innovate further in step 1 through a precision medicine approach.
Once inflammation is controlled, symptoms typically improve, but patients are not cured. This is where Step 2 comes in – the ability to reset immune memory. Earlier this year, Professor Georg Schett published data in the New England Journal of Medicine on 15 patients with severe SLE (8 patients), idiopathic inflammatory myositis (3 patients), or systemic sclerosis (4 patients) who received a single infusion of CD19 chimeric antigen receptor (CAR) T cells after preconditioning with fludarabine and cyclophosphamide (link here). This research provides additional rationale for further controlled clinical trials.
(For an inspirational video on CAR-T, please see this link to a video from BMS entitled “Science Firsthand: Translating the promise of cell therapy.”)
Beyond CD19-mediated immune reset, there are other potential mechanisms to reset immune memory. For example, many autoimmune diseases are driven by the loss of tolerance to specific self-antigens. When these autoantigens have been clearly identified, it may be possible to re-tolerize by presenting these antigens to the immune system in a non-inflammatory and tolerogenic environment. At BMS, we recently announced a partnership with Repertoire Immune Medicines to develop tolerizing vaccines for up to three autoimmune diseases.
I want to emphasize that achieving the ambition of Step 2 approaches has been elusive. Until recently, functional cures through resetting immune memory were considered only theoretically possible. Now, however, immune reset appears possible in practice.
Finally, step 3 promotes immune homeostasis and tissue repair. This is an important step after immune reset, as even after autoreactive cells are eliminated, autoantigens may still be present to re-initiate an autoimmune process. By promoting T regulatory cell expansion and repairing damaged tissue, it may be possible to prevent autoimmunity from every appearing again in that patient.
As a former practicing rheumatologist, I know what a burden chronic therapy can be. Now, through sequential immunotherapy, I hope that one day clinicians can offer patients with autoimmunity not only a diagnosis and medicines to treat their symptoms, but also medicines that provide transformational efficacy, durable remissions and, ultimately, cures.
Addendum:
I had the opportunity to catch up with Dennis recently, and he elaborated further on the sequential immunotherapy concept. He pointed out that even after a successful immune reset (Step 2), many patients will still have a genetic predisposition for autoimmune disease. This opens opportunities for discovering precision drugs that act prophylactically to prevent relapses (Step 3) by leveraging human genetics to identify targets with high probabilities of success. He mentioned recent work where immune reset was achieved in atopic diseases with a BCMA x CD3 bi-specific antibody to deplete plasma cells, followed by IL-4Rα blockade to prevent new B cells from class switching to IgE.

